Fireproof Microcapsules
 Product Matrix
Product MatrixNotice
Welcome to follow this project!🤝
Our team is willing to discuss and cooperate with relevant experts, engineers and technicians in depth on the development and industrial application of fireproof microcapsules.
For the latest progress, please check my related post.
Project Profile
Introduction
As an energy carrier that can realize the mutual conversion of chemical and electrical energy, lithium-ion batteries are widely used in portable electronic devices, smart grid energy storage, new energy vehicle power systems, etc. due to their high operating voltage, good cycling performance, no memory effect, high specific energy, good stability and long service life.
At present, lithium-ion batteries are still not intrinsically safe. In EVs (electric vehicles) and ESS (energy storage systems), hundreds of batteries are usually used in series and parallel in order to meet the requirements of operating voltage and power, and they face problems of extrusion, breakdown, temperature shock, overcharge and short circuit under long time use. Once the battery is under such conditions, the battery may generate a large amount of heat, which triggers a chain reaction of internal electrolyte and electrode materials (leading to electrolyte and positive electrode decomposition), which in turn leads to thermal runaway and may develop into a large-scale explosion and fire accident.
A common strategy to prevent thermal runaway in Li-ion batteries is to modify the surface of the diaphragm using inorganic particles (e.g., ceramics and titanium oxide) that are highly thermally stable to improve the heat resistance of the diaphragm. The compatibility of inorganic particles with the diaphragm is usually poor and requires the use of a binder, however the use of a binder will inevitably reduce the porosity of the diaphragm and significantly increase the thickness of the separator, which is not conducive to efficient ion transport in the separator.
Coating inorganic particles on the diaphragm to give it high thermal stability or developing new diaphragms, which is based on the concept of passive protection against thermal runaway of the Li-ion batteries. How to inhibit the initial thermal runaway of Li-ion batteries from the source and block the internal chain reaction of thermal runaway of Li-ion batteries, thus ensuring that the diaphragm will not be damaged when thermal runaway occurs, is a major challenge for current research.
Fireproof Microcapsules
The use of fire-extinguishing agents to suppress the lithium battery deflagration is a good choice. However, the existing fire-extinguishing agents halogenated alkane 1301, carbon dioxide, heptafluoropropane, can only extinguish the open fire, but can not fundamentally inhibit the fire, which leads to the re-ignition of the battery, and does not have the dual function of cooling and fire-extinguishing.
Moreover, complex fire fighting facilities will increase the weight or volume of the energy storage system, which is not absolutely reliable in the case of thermal runaway. Therefore, the selection of suitable fire-extinguishing agents and the design of new fire-extinguishing devices are important research areas for the large-scale application of lithium-ion batteries.
Therefore, considering the shortcomings of most halogenated hydrocarbon extinguishing agents such as high cost, low boiling point, difficult storage, use of complex equipment fittings, and high pressure required for injection, microencapsulation of halogenated hydrocarbon extinguishing agents is a good and feasible solution. So, what can be used as the core material of microcapsules to effectively achieve fire-extinguishing and flame retardant function?
It was found that fluorinated derivatives can stop the chain reaction that occurs during lithium-ion battery combustion and have no other negative effects on electrical equipment, such as perfluorocaproone (NOVEC 1230), a green fire-extinguishing agent that contains no bromine or chlorine, has an almost zero ozone depletion value, and degrades completely in the atmosphere within 1 to 2 weeks.
Noteworthy, at low concentrations, perflourohexanone can instead enhance combustion. Therefore, according to the actual lithium-ion battery ignition conditions, it is necessary to select a suitable high boiling point, high heat of vaporization and high specific heat composite fire-extinguishing agent (fire-extinguishing agent + coolant) in order to ensure the fire-extinguishing performance while taking into account the cooling capacity.
However, perfluorhexanone is difficult to microencapsulate because its inactive nature makes it difficult to dissolve or be dissolved into other substances, making it difficult to synthesize microcapsules by chemical methods. In addition, the high density and volatility of perflourohexanone make it difficult to be microencapsulated by other general techniques.
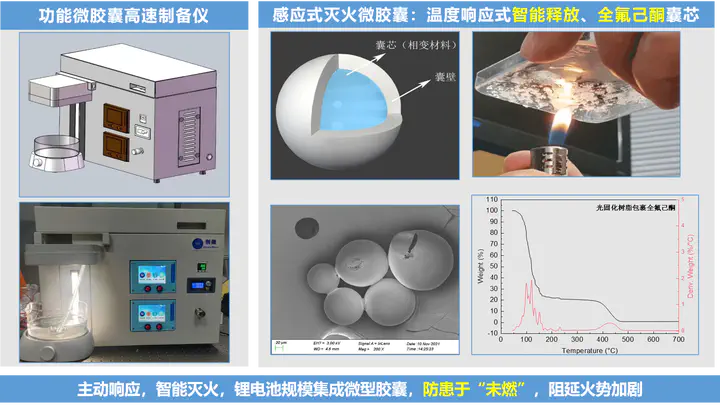
To address these issues, our team encapsulated perflourohexanone through actively excited microfluidic technology, which not only isolates it from the external environment to protect and store the extinguishing agent, but when the ambient temperature rises (~80°C), perflourohexanone, the core material, absorbs heat and evaporates, rising through the outer walls of the microcapsules, thereby releasing and inhibiting further chain reactions and eliminating the fire before it is too late.
In the pre-exploration, we have not only verified that the fireproof microcapsules developed by our team can remain stable in the electrolyte (30 days), but have also conducted experiments on small batteries (aluminum-plastic film batteries, 3.2V-40Ah) and achieved good fire-extinguishing results.
Notes
The content of this article is derived from a number of literature, please correct any inaccuracies.